If you produce, manufacture, store, or sell food, you may be required to write a Hazard Analysis and Critical Control Points (HACCP) Plan specific to your commodity and process. This written HACCP plan may be required by the Department of Health or other agency for you to receive a food license or permit.
What is HACCP?
It is a globally recognized, industry-standard comprising of 7 key principles used to review your ingredients, process, facility, and practices to identify biological, chemical, physical hazards and establish Critical Control Points to prevent, reduce or eliminate the significant hazards. Long story short, it is a scientifically valid plan that assures the food you handle is consistently safe with the goal of protecting the consumer.
What are the 7-key principles?
Principle 1. Conduct a Hazard Analysis
The process of identifying the hazards associated with your product, such as:
Food hazards fall into three categories:
Principle 2. Identify Critical Control Points
Each significant hazard identified in the Hazard Analysis above will need a control to reduce (to an acceptable level), prevent, or eliminate the risk. Just a reminder, some industries are regulated, like juice and canned items. Always ensure you are abiding by any commodity-specific regulations.
Principle 3. Critical Limits
A critical limit is a scientifically specific value or value range, such as a temperature or the amount of time needed for the Critical Control Point to effectively manage food risks. Be cautious not to confuse this with quality processes. For example, you may prefer to eat your meat a little on the pink side. To control pathogens in a meat patty, the internal (center) of the patty must reach 151*F for 41 seconds to be safe for consumption (according to research). The critical limit is vital to ensure you are producing a safe, wholesome product.
Principle 4. Monitoring of Critical Control Points
At this point in the process, you have identified all the hazards associated with your ingredients, production process, facility, etc., and identified the critical control points to manage the risks and the critical limits required to ensure the threats are controlled effectively and your food will not harm the consumer. Now we must implement a process to ensure every single food item you produce is within control and documented. We accomplish this by developing monitoring and verification procedures. Let’s start with the What, How, When, and Who.
Records prove that you monitor your critical control points and serves as historical proof to protect you if an issue ever arises. See the example below for a CCP monitoring procedure.
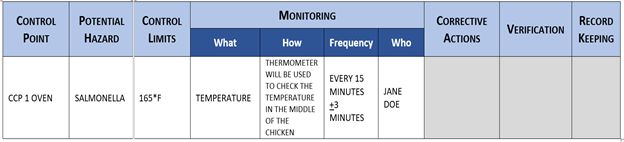
Principle 5: Corrective Actions
This principle addresses what happens if something goes wrong. What if the cooling system in storage fails or the sanitizer level is too low? Corrective Actions should assist in determining the root cause of the issue, the steps to take to correct the deviation, an evaluation of the food product impacted by the nonconformance, documented records of the actions taken to resolve the issue, and the outcome of the impacted food such as rework, destroy, etc.
Principle 6: Verification & Validation Procedures
Verification of the HACCP Plan is the activities to ensure the monitoring procedures are working as intended, such as calibrating thermometers to provide an accurate reading, observing an operator performing the monitoring procedures correctly, etc.
Validation of the HACCP Plan is gathering scientific and technical research and studies to ensure the controls and limits effectively control the identified hazards.
Principle 7: Record Keeping
Records fall into two categories, supporting documents and operational records. The “Supporting Documents” will include the names and contact information for your HACCP team members and any outside consultants used during the steps above. The records consist of meeting minutes, decisions made throughout the process, and reasons for decisions made. Supporting scientific and technical data, manufacturers’ information, industry trends, or historical industry issues will also be part of the supporting documents.
“Operational Records” are the verification and monitoring activities performed each day or every time food is produced. Storage (including length of time) of records should comply with federal regulations. Most of the time, records are stored for the length of the shelf life of the product plus one year.
Why does the Department of Health require a plan?
HACCP is a globally recognized industry standard, not a regulation. In the United States, most food handlers must operate with a Food Safety Plan under the “Preventive Controls Rule,” which requires a Hazard Analysis to be completed specific to your operation, and preventive controls must be identified and put in place. The state, county, and local regulators, also follow these requirements to protect public health.
What are the benefits of having a Plan?
In addition, to be compliant with regulations, the process of conducting the Hazard Analysis means you will examine each step of your operation, including your supply chain, each ingredient, and additive, processing equipment, chemicals used, the intentional and unintentional introduction of hazards, etc. Once identified, you will use scientifically validated controls to manage your food risks at each step of the process. The benefit is the systemic approach to ensuring you are producing safe, wholesome food for your customers and protecting the integrity of your brand.
Should I use Global Food Safety Consultants (GFSC) to write my plan?
Proper identification of hazards and control can be scientific and challenging if you do not have formal education or experience in the food sciences. GFSC is a group of professional food safety experts that design and integrate food safety programs into your business so you can get back to doing what you love most.
If you or your company need assistance with writing a HACCP Plan, Global Food Safety Consultants, your food safety partner, can help.



